Textiles, as a necessity in people's daily life, are important in their antibacterial finishing. Wool fiber fabrics have the advantages of smooth touch, good moisture absorption and warmth, but the microporous structure and keratin components of wool fibers provide moisture and nutrition for the growth of bacteria. People put on higher requirements for the sanitation of wool fabrics while wearing comfort. In recent years, the antibacterial finishing of wool has become more and more mature. The development of antibacterial wool is of great significance to meet daily needs, expand the application space, and promote the progress of the textile industry. The antibacterial finishing of wool fibers mainly adopts a post-finishing process, that is, it imparts special functions to fabrics by loading various antibacterial agents.

1 Wool fiber antibacterial finishing mechanism
Different antimicrobial agents have different roles and objects of action. The mechanism can be summarized in two categories: The first is the use of cationic groups in antimicrobial agents to adsorb negatively charged bacterial cell walls through electrostatic interactions. They can disrupt cell wall structure, inhibit protein synthesis, inactivate cell synthetases, and can also be altered The permeability of the cell wall makes the intracellular solution seep out to achieve the antibacterial effect; the second is to use the small size effect and photochemical activity of the nanomaterial to generate strong oxidizing groups and react with microorganisms under the photocatalytic conditions to cause bacterial death.
2 Wool antibacterial finishing method
Based on the antibacterial mechanism of commonly used antibacterial agents, antibacterial finishing of wool fibers can be classified into inorganic metal ion antibacterial finishing, organic antibacterial finishing, natural antibacterial finishing and nanomaterial antibacterial finishing.
2.1 Inorganic metal ion antibacterial finishing
In the antibacterial finishing process, people have long used metal salt compounds to treat fabrics and give fabrics special functions. Wool fiber structure is special, contains a variety of amino acid structures, amino acid residues on the amino acid, carboxyl, hydroxyl and other groups that can adsorb all kinds of metal ions.
Studies have shown that the adsorption of metal ions by wool fibers is affected by the ion species, concentration, solution pH, treatment time and temperature, among which the pH value has the greatest effect. Taking silver ions and copper ions as examples, under acidic conditions, Ag+ and Cu2+ are mainly combined with carboxyl groups, and when the pH value is lower than the isoelectric point of wool (4.2 to 4.8), the surface of the fiber is positively charged, and electrostatic repulsion with Ag+ and Cu2+ is more Weak; When the pH value is above the isoelectric point, the surface of the wool fiber is negatively charged, and the electrostatic attraction between the two causes an increase in the amount of adsorption. In the basic ammonia-containing condition, silver ions and copper ions are bound to the nitrogen-containing side groups of the wool through the form of silver ammonia and copper ammonia complexes, and the adsorption capacity is improved compared to the acidic environment. However, the keratin structure of wool changes in strong alkali solution, and the amino acid structure such as cystine residues decomposes, which affects the stability of the wool fiber, and the visual performance is that the fiber strength is reduced and the handle is rough.
In spite of this, the binding fastness and antibacterial durability of metal ions and wool are still flawed. Some scholars have solved this problem by loading metal ions into wool fibers through cross-linking. Commonly used crosslinking agents include tannic acid and seaweed. Sodium and so on.
2.2 Organic Antibacterial Finishing
At present, various types of organic antibacterial agents represented by quaternary ammonium salts occupy a dominant position in the market. Quaternary ammonium salts, biguanides and imidazoles and other medicinal antibacterial agents are gradually applied to the antibacterial finishing of wool products.
Quaternary ammonium compounds can be divided into single long chain quaternary ammonium salts, double long chain quaternary ammonium salts and complex quaternary ammonium salts. Factors that determine the antibacterial properties of quaternary ammonium salts mainly include molecular weight and alkyl chain length. According to reports in the literature, the molecular weight of the quaternary ammonium salt increases, the charge density increases, and the antibacterial activity increases. At least one of the four branches of N+ is between C8 and C18, and the quaternary ammonium salt has good antibacterial activity. . Ordinary quaternary ammonium salt has poor binding ability with fibers, and as a dissolution-type antibacterial agent, it is easily eluted and enriched in human body, and thus has certain limitations. At conditions greater than the isoelectric point of the wool, the cationic group can generate electrostatic attraction with the negatively charged wool fibers, and the alkyl chain can also hydrogen bond with the side groups of the wool. However, due to its large molecular weight, it is easily soluble in water and easily elutes, and the combination with fiber is not strong and durability is not strong. Therefore, some scholars improve the adsorption capacity and binding fastness of quaternary ammonium salts by modifying wool fibers.
The diterpene salt, originally used as a medical chemical, is a major component of many disinfecting solutions and was gradually used in fields such as food, cosmetics, and textiles. ICI developed the double oxime structure as an antimicrobial agent for textiles. Its main component is polyhexamethylene biguanide hydrochloride (PHMB). Unlike traditional quaternary ammonium salts, the safety of polyhexamethylene biguanide hydrochloride (PHMB) Sex has been widely accepted by many people and many scholars have confirmed their non-toxicity to their organisms. Polyhexamethylene biguanide hydrochloride has good chemical activity, and the positive charge is electrostatically adsorbed with the groups in the wool fiber, and is formed on the surface of the wool and deposited on the surface of the wool. PHMB can be formed with the aid of a cross-linking agent. The co-agent-wool system is more excellent in antibacterial durability and can also improve some of the physical properties of wool fibers.
2.3 Natural antibacterial finishing
Extracts and refines natural antibacterial agents from animals and plants, with good environmental compatibility and antibacterial activity, mainly including chitosan, ε-polylysine and lysozyme. Among them, chitosan has become the most popular natural antibacterial agent for its safety, non-toxicity and simple source.
Under acidic conditions, the amino acid is converted into amino cations, and chitosan becomes a positively charged macromolecular polysaccharide, which is the main reason for the antibacterial effect, and it also provides the potential for electrostatic adsorption with wool fibers. However, similar to most other cationic antibacterial agents, such a combination cannot achieve long-lasting antibacterial effects. Many scholars have modified or compounded chitosan to improve the antibacterial effect of chitosan antibacterial agents, including quaternary amination, deuteration, carboxyalkylation modification or compounding with nano titanium dioxide, nano silver and so on.
Ε-polylysine is an antibacterial agent that people simulate biological structures, prepared by chemical synthesis or microbial fermentation. The ε-polylysine forms a positively charged cationic polymer in solution, and ε-polylysine can be grafted to the wool fiber by means of the catalytic cross-linking of transglutaminase (MTG). The amide group in MTG reacts with ε-polylysine and lysine residues, primary amino groups in wool fibers, so that the two form a stable covalent crosslink.
Lysozyme has a wide range of sources and can be isolated and extracted from animal body fluids and plants. It is in the free state in the aqueous solution, it is difficult to combine with the wool fiber, the stability and controllability of the lysozyme can be improved by the immobilized enzyme process, and the free enzyme is “fixedâ€, which is necessary for the antibacterial finishing of the wool by lysozyme. Link. Huang Dong and Shao Xiaojuan directly immobilized lysozyme on wool fibers and imparted antibacterial properties to fabrics by MTG-catalyzed crosslinking of lysozyme.
2.4 Antibacterial Finishing of Nanomaterials
Nanomaterials are used in many fields due to their unique surface effects, size effects, and photochemical activity. Researchers have put nano-silver, nano-titanium dioxide, and nano-silica nanomaterials into wool fibers to achieve anti-bacterial and anti-UV effects. Among them, the antibacterial mechanism of nanometal oxides is different from that of traditional cationic antibacterial agents. Under photocatalytic conditions, nano metal oxides undergo electron transitions and generate positively charged holes, and electrons and holes generate strong oxygen radicals with oxygen in the environment. The pellet reacts with microorganisms, causing it to die. The antibacterial mechanism of nanosilver is the combined action of metal ion dissolution mechanism and photocatalytic mechanism.
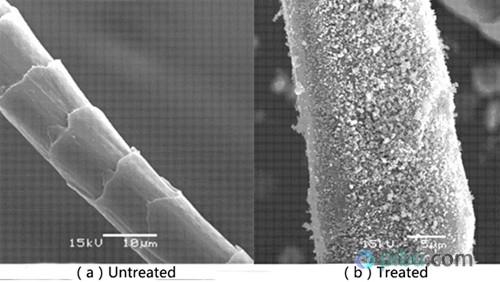
Sol-gel method is the most commonly used method for preparing related nanomaterials. Nano antibacterial agents can be loaded onto wool fibers through a traditional roll roasting process. When colloidal finishing fabrics often form an antibacterial layer on the wool surface, and nanomaterials and wool are usually combined with weaker molecular forces such as hydrogen bonds and van der Waals forces, so the finishing process is often accompanied by fiber modification to improve the binding of the two. degree. Commonly used modification methods include ultraviolet irradiation, plasma treatment and chemical reagent modification.
Studies have shown that the sol-finished wool fabrics have problems such as poor hand feel, decreased strength, and impaired original fabric style. Moreover, the organic solvents and strong acid media in the nano-sol preparation process are flammable and toxic, which is detrimental to actual production. Some scholars use in-situ preparation methods to generate nano-antibacterial agents in the internal pores of wool fibers. This process is not only simple, but also avoids the problem of impaired physical properties of the fabric caused by the surface coating fixation, and the washing performance is also improved.
3 Conclusion
At present, the types of antibacterial agents on the market are still limited, and due to biological toxicity and other issues, some antibacterial agents have not been widely accepted by people. The development of domestic antibacterial agents started late and is still in its infancy. Many reports on new antibacterial agents are still in the laboratory stage and it is difficult to adapt to large-scale production. Antibacterial finishing of wool should always focus on the requirements of broad-spectrum sterilization, biological low toxicity, durability, and maintaining the original style, and it should be in the direction of environmental protection, comfort, and high efficiency. While paying attention to the antibacterial properties of antimicrobial agents, scholars should also focus on solving the problem of the combination of antimicrobial agents and fibers. In the future, new antibacterial agents will certainly add to the lives of people with simple finishing processes, long-lasting antibacterial properties, good biocompatibility, and improved fabric handling performance.
For more content, please follow this site
ZHE JIANG LUO RUI IMPORT CO.,LTD. , https://www.lrknitted.com